Top Software as a Medical Device companies in the US
To be a qualified Software as a Medical Device company in the US, the product must first be approved by FDA and HIPAA standard. This is our first criterion when compiling this list.
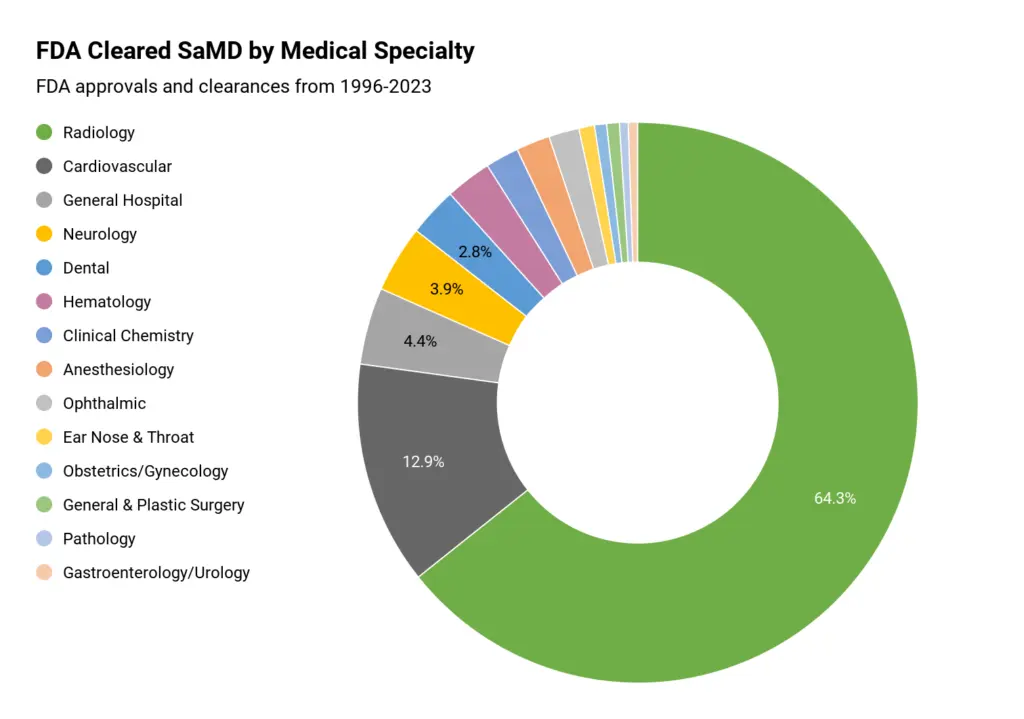
At the same time, we select both product and service-based company, where you can either purchase a SaMD product for your facilities, or work with a vendor to customize your own. Let’s learn about them!
| Company | Type | Best for | Rate |
|---|---|---|---|
| Digital Diagnostics | Product | Diabetic retinopathy | 4.2/5 (Google) |
| Tempus | Product | Oncology | 3.4/5 (Google) |
| Paige | Product | Oncology | 4.3/5 (Google) |
| Bright Insight | White paper | Disease management | n/a |
| Scnsoft | Custom service | AI-based SaMD | 4.8/5 (Clutch) |
| Orthogonal | Custom service | Diagnostics SaMD | n/a |
Digital Diagnostics
As the first SaMD company to meet FDA standard for an AI diagnostic platform, Digital Diagnostics stands out with Luminetics – a diabetic retinopathy detector. This SaMD product analyzes retina images of diabetic patients and identifies early signs of vision loss.
The best part? The process is entirely automated by AI without physician evaluation in less than a minute, immediately at the point-of-care.
A fast and automated diagnosis is the future standard of care. Digital Diagnositics is currently one of the leaders, as they will continue to expand and research new SaMD solutions.
Other information:
- Location: Coralville, Iowa
- Company size: 51-200 employees
- Security and Compliance Certification: HIPAA, FDA
- Best for: Front-line care providers, Primary care, Eye care practioners, Payor Community
- Customer: Tarzana Treatment Centers, Cahaba Medical Care, Johns Creek Primary Care
Tempus
Tempus is one of the biggest SaMD companies in the US with $1.3 billion in funding. Focusing on providing precision medicine and treatment, they offer many AI-based tools for Oncology, Neurology, Cardiology, Radiology, and Dermatology.
However, this leading SaMD company is best known for their work in Oncology. For example, their Digital Pathology uses AI to find actionable markers in specimens. From there, the system can determine if the patient has the potential for clinical trial. You can discover their full product listing here.
Much like other peers, they rely on AI and vast clinical and molecular data to make data-driven analysis. Furthermore, the team also collab with 200+ biopharma to drug research and discovery.
Other information:
- Location: Chicago
- Company size: 1000-5000 employees
- Security and Compliance Certification: HIPAA, FDA
- Best for: Oncologist, Primary care, Hospitals, Psychiatrist, Dental
- Customer: 65% of US academic medical centers, 95% of top 20 pharma oncology
Paige
Another AI-based Software as a Medical Device company, Paige, focuses on detecting up to 40 cancer types in prostate, breast and colon. They also provide tools to assess phenotypic changes and genetic alterations in cancer, as well as support pathologists in pancancer cases.
How do they do it? Well, the team uses 25 million H&E-stained whole-slides images and works with many labs to attain a large data system for AI training. This ensures the highest accuracy and eventually reduces 70% of cancer detection errors.
Other information:
- Location: New York
- Company size: 150 employees
- Security and Compliance Certification: ISO 13485, FDA
- Best for: Labs, Pathologists, Oncologist, Primary care, Hospitals
- Customer: 800+ labs and healthcare facility
Bright Insight
Starting as a biopharma and medtech digital health solution provider, Bright Insight soon realized Software as a Medical Device will be the backbone of next-generation healthcare. From there, they provide white-paper solutions in creating and managing SaMD for disease management, with a high focus on device management, data integration and regulations compliance.
The company hopes to provide rapid development of medical apps and devices across therapeutic areas, including Cardiovascular, Gene therapy, Diabetes, Immunology, Oncology, Mental Health, and Respiratory.
Other information:
- Location: San Jose
- Company size: 200-500 employees
- Security and Compliance Certification: HIPAA, FDA, GDPR, ISO 27001, HITRUST CSF
- Best for: Facilities want to customize their SaMD solution
- Customer: Woebot Health, Rhapsody, Cooley, Xealth
Scnsoft
With better flexibility, customizing your SaMD can be a great way to improve your quality of care. Scnsoft, with 19 years of experience in building healthcare solutions, is a must-have partner on your selection list.
They make sure your SaMD is functionable, secure and FDA-cleared. The team are also well-versed in using emerging technologies such as AI/ML, advanced analytics to established ones such as IoT, cloud, and teal-time tracking.
What you can build with this Customed Software as a Medical Device company:
- AI-based disease treatment and planning
- ML-based prescription recommendation system
- AI-based diagnosis via medical image recognition
- Personalized treatment
- FDA Level 1 to Level 4 SaMD solution
Scnsoft once worked with a genetic screening kit provider to build Laboratory Diagnostics Software that can quickly interpret test results. This SaMD is built in 7 months and delivered with IVDR, MDR, IEC, EC, and ISO standard.
Other information:
- Location: Texas
- Company size: 750+
- Security and Compliance Certification: ISO 13458, ISO 9001, ISO 27001, HIPAA, FDA, HITECH
- Best for: Medical device manufacturer, Healthtech startups, Healthcare providers, Pharmaceuticals company, Lab research, Healthcare NGOs
- Customer: 150+ healthcare clients
Orthogonal
While healthcare is only one of many industries Scnsoft serves, Orthogonal, on the other hand, focuses solely on building medical device software.
This allows them to effectively navigate the industry’s challenge and understand every intricacy. With over 10 years of experience, they make sure your SaMD solution is scalable, powerful, and qualified, following Agile methodology.
What you can build with this Customed Software as a Medical Device company:
- AI-based SaMD
- Mobile app, desktop app, cloud-based app
- SDK platform integration
- Digital diagnostics, digital biomarkers, digital therapeutics
- Class I, II, III connected medical devices and active implantable
Many clients, including CEO, CTO and Head of Engineering in Healthcare companies praised Orthogonal for their adoption of modern engineering in SaMD development.
Other information:
- Location: Chicago
- Company size: 50-100 employees
- Security and Compliance Certification: ISO 13458, ISO 14971, IEC 62304, FDA, GDPR, MDR, HIPAA
- Best for: Venture-backed startups, Medical devices company, Pharmaceutical company, Medtech enterprise
Top SaMD Companies in Europe
The regulatory landscape and risk classification for Healthtech in Europe is different from the US. For example, the EU does not rely on FDA but instead uses MDR standard (European Union Medical Device Regulation) and CE certification. At the same time, they mostly refer SaMD as “medical device software”, which for the US might be the wrong term.
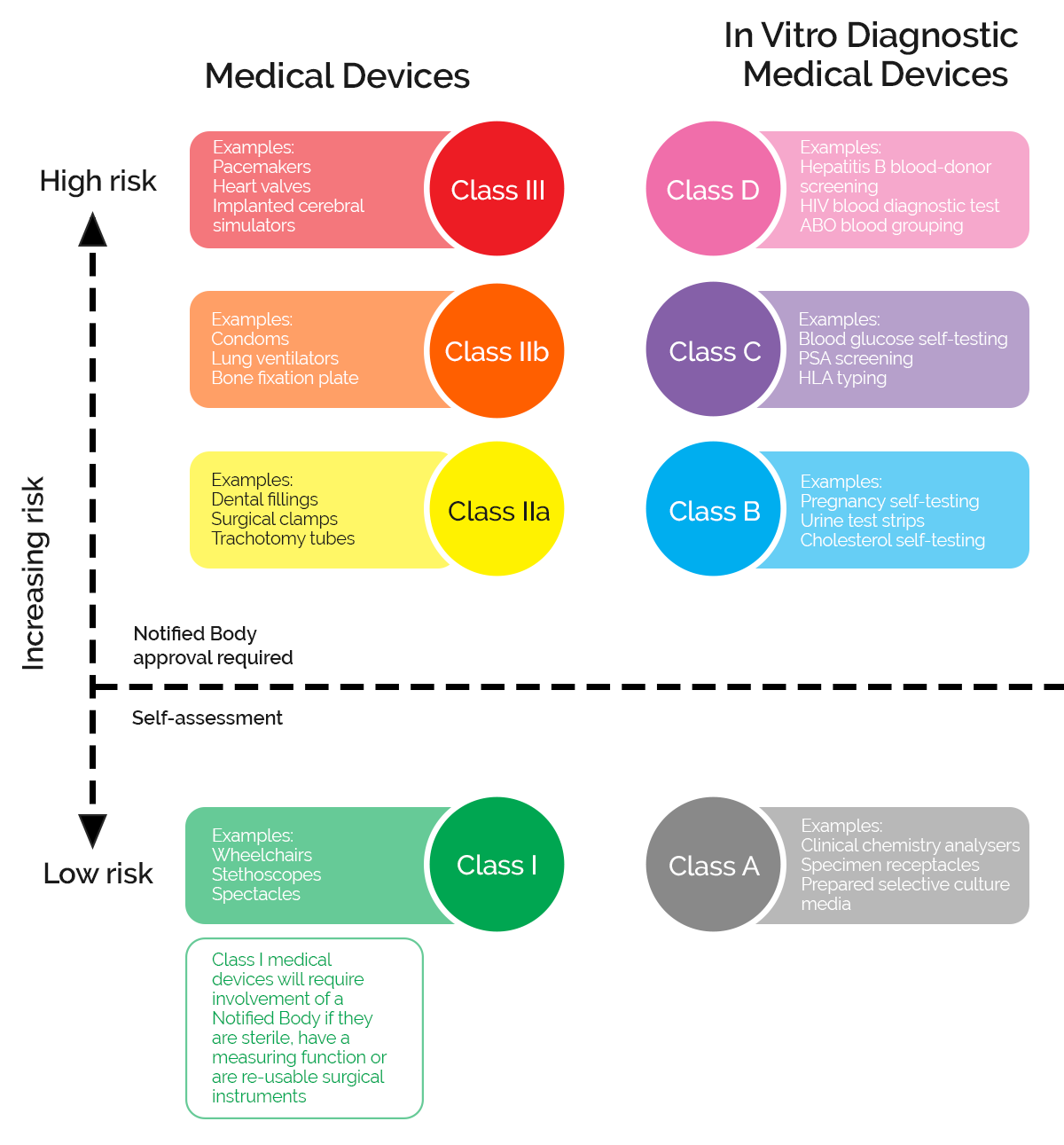
This means that SaMD products and services based in the US might not always be the best option for countries in Europe. In the list below, we selectively pick Software as a Medical device company from Europe and meet the EU standard.
| Company | Type | Best for | Rate |
|---|---|---|---|
| Mindmaze | Product | Brain recovery | n/a |
| Roche | Product | Diagnostics SaMD | n/a |
| Cambridge Cognition | Product | Cognitive SaMD | n/a |
| S3 Connected Health | White paper | DTx | 4.7/5 (Google) |
| HTD Health | Consulting | Medtech | n/a |
| Apotech | Consulting | Medtech | 5/5 (Google) |
| Andersen Lab | Custom service | Medtech | 4.9/5 (Clutch) |
| Zuehlke | Custom service | Data-driven SaMD | n/a |
Mindmaze
Best known for their work in neurotechnology, this DTx and SaMD company leverages AI, virtual reality, motion analytics, bio-sensing and cloud to build evidence-driven assessment and treatment for brain recovery.
With 20 years of experience, Mindmaze attains a portfolio of 7 solutions and therapy games, treating Alzheimer, Parkinson and aging patients, traumatic brain injuries, and neurological diseases.
For example, their most notable solution is the MindMotion, used by 90 leading recovery centers worldwide to support telerehabilitation. The system mimics the movement of physiotherapy so that patients can practice at home and anywhere. Meanwhile, solution like Brain Vitals actively measures longitudinal changes in cognitive function.
Other information:
- Location: Switzerland
- Company size: 150-300 employees
- Security and Compliance Certification: MDR, HIPAA, CE, ISO 13485
- Best for: Physiotherapy center, Patients, Recovery center for athlete, Neurorehabilitation center
- Social proof: 100,000+ sessions completed, 600+ papers and studies
Roche
This Swiss-based Healthtech company not only focuses on discovering new medicines but also creating state-of-the-art diagnostics and digital health solution.
For the past 125 years, Roche prioritizes effective personalized care and healthcare innovation, and from there, actively creates many SaMD diagnostic solutions. These include in-vitro tests, laboratory-based diagnostic instruments, and evidence-based digital health solutions.
Some notable ones are a cancer screening and treatment monitoring system and a blood sugar tracking and management for diabetes.
Other information:
- Location: Switzerland
- Company size: 10,000 employees
- Security and Compliance Certification: MDR, HIPAA, CE, FDR, ISO 13485
- Best for: Pharmaceuticals, Lab, Primary Care, General Hospitals, etc
Cambridge Cognition
As their name implies, this SaMD company specializes in brain health and digital cognitive assessment. Cambridge Cognition has built many healthcare tools and decentralized clinical trial platforms for the past 30 years.
For example, CANTAB, their best work, can accurately assess Alzheimer, ADHD, depression, schizophrenia, and Multiple Sclerosis. You can use CANTAB in the clinic or remotely through its web-based testing. Cambridge also provides voice biomarkers built with ASR that have up to 98% accuracy.
Other information:
- Location: England
- Company size: 51-200 employees
- Security and Compliance Certification: MDR, HIPAA, CE, FDR, ISO 13485
- Best for: Drug development company, Healthcare organizations, Neuro research institutions
- Social proof: 3000+ peer reviewed papers & articles
S3 Connected Health
Existing SaMD might lack a certain flexibility, but building one from scratch can be intimidating. This is where this Software as a medical device company steps in.
S3 Connected Health is best known for their Affinial platform, which allows healthcare providers to customize and operate DTx, chronic and therapy management solutions to their liking.
The platform strongly relies on IoT technology and real-time data from connected medical devices and other sources to build SaMD solutions for Pharma and Medtech across 20+ therapy areas.
S3 Connected also partnered with Biogen to build TrackSMA – a clinical assessment for spinal muscular atrophy. This solution was first launched in APAC but now has been used worldwide by thousands of patients.
Other information:
- Location: Dublin
- Company size: 300+ employees
- Security and Compliance Certification: MDR, HIPAA, CE, FDR, ISO 13485
- Best for: Drug development company, Healthcare organizations, Neuro research institutions
- Customer: 30+ leading pharma and medtech customers
HTD Health
Based in Poland and New York, this IT company has been customizing Health solutions for companies in the EU and US for the past 7 years. You can trust HTD for Software as a medical device development, as the team promises to deliver with technical quality and regulatory fit.
Adopting Agile in development, HTD Health will guide you through planning to final deployment, ensuring your SaMD solution is safe and high functioning.
What you can build with this Customed Software as a Medical Device company:
- Class I, II, III medical device development
- SaMD for Medtech
HTD Health helped a client build a wireless, handheld ultrasound device that can be used through a mobile app. The software meets many regulatory standards such as FDA, ISO, to 510 (k) approved.
Other information:
- Location: Poland
- Company size: 200-500 employees
- Security and Compliance Certification: FDA, ISO 13485, ISO 27018, ISO 72001, SOC Type I and II, GDPR
- Best for: Healthcare and Pharmaceuticals of all types
- Customer: Boston Children Hospital, Zus, Johnson & Johnson, Athena Health
Apotech Consulting
If you are looking for a consultant on how to adopt and build a Software as a medical device solution, Apotech can be the right choice. Strong in AI and ML, they can give you the right guidance in harnessing SaMD for diagnostics, monitoring and treating patients.
Not only that, but they also support pre-market strategy development, regulatory submissions, and clinical evaluation of your SaMD. Throughout the years, they have created a strong portfolio with over 50% of clients coming from Medtech or looking for SaMD solutions.
A prime case study is when Apotech worked with Deciphex, an SaMD for pathology, in suggesting a regulatory framework and navigating FDA/ IVDR submission challenges in the UK
Other information:
- Location: London
- Company size: 50 employees
- Security and Compliance Certification: ISO 13485, ISO 14971, ISO 12207, FDA, HIPPA, MDR, CE, QMSR
- Best for: Healthcare and Pharmaceuticals of all types
- Customer: Phillips Healthcare, Medtronic, Pfizer
Andersen Lab
Unlike other Custom Software as a medical device companies on this list, Andersen solely serves Medtech companies. Being a global IT company, you can trust them for their in-depth technical skill. In Medtech, they possess a strong understanding of the industry and how to navigate regulation problems, after working on many projects.
What you can build with this SaMD company:
- SaMD specialized data structure
- AI-based SaMD
- Usability engineering and UI/UX services
- SaMD development of all types
During Covid-19, Andersen worked with a client to develop a reliable Web-based tool for Covid-19 Diagnostics. They also created a digital platform for obstructive sleep apnea patients.
Other information:
- Location: Germany, Poland, UK
- Company size: 150+ employees
- Security and Compliance Certification: HIPAA, GPDR, ISO, MDR
- Best for: Medtech company
- Customer: 4Medical, Chron Well, Emfit
Zuehlke
Zuehlke is a global IT and software engineering company based in Switzerland. Despite working with many industries, the team stands out in supporting Healthcare, Pharma and Medtech clients.
From custom digital healthcare tools to whitepaper SaMD solutions with AI/ML, IoT, and data analytics, Zuehlke can do it all.
The team once directly worked with the UK government for the development of NHS Covid-19 app which prevented over 1 million potential infections. Another notable case is the automated classification of diseases.
Other information:
- Location: Switzerland
- Company size: 1000 – 5000 employees
- Best for: Healthcare organizations of all types
- Customers: Inselgruppe, STS Hospital Group, City of Vienne
Top SaMD Companies in Asia and Australia
Compared to the US or EU, the software as a medical device market in Asian and Australia is still growing strong, but many countries have yet to develop security standard and quality regulation for SaMD.
At the same time, some regions such as ASEAN may lack a well-versed developer to build an effective SaMD. This list contains some of the most prominent names.
Title ảnh: Quick comparison of the top SaMD companies in Asia and Australia.
| Company | Type | Best for | Rate |
|---|---|---|---|
| Synodus | Custom service, White label | SME health facility | 5/5 (Clutch) |
| Guardan Health | Product | Oncology screening | n/a |
| Harrison | Product | Radiology, Pathology | n/a |
Synodus
Having worked with many healthcare providers across APAC in customizing Healthtech solutions, Synodus is a great contender. Strong in Cloud, IoT, and Data analytics, Synodus sure brings a dedicated touch to your SaMD, making it fit whatever you request.
Based in Vietnam, Synodus understands every intricacy of healthcare in APAC. We also make sure every solution is HIPAA, GDPR, and FDA-cleared, putting the highest priorities on quality and security to meet global requirements side by side local ones.
What over 30+ facilities with around 10,000 healthcare experts love most about us:
- A cost-effective approach that can save up to 65% of initial cost
- A fast delivery that can deploy your SaMD 3x times faster
- In-depth knowledge of healthcare and technology
- Well-versed in the use of IoT and Data for healthcare
If you are a small to mid-size facility, Synodus is your best fit for custom and data-driven screening and diagnosis solutions. We can also build HIS, EMR/EHR, or other digital health solutions, creating a strong ecosystem for your facility.
Other information:
- Location: Vietnam
- Company size: 250 employees
- Security and Compliance Certification: HL7, HIPAA, FHIR, GDPR, ISO 27001, ISO, FISMA, SAFER
- Best for: Healthcare and Pharmaceuticals of all types
- Social proof: 70% boost in operational performance, 30% increase in patient satisfaction
Have an SaMD in mind but don’t know where to start?
Guardant Health
Comes from the US, this software as a medical device company has expanded their touch to many Asian countries. You can reach out to Guardant in Singapore, China, Japan, and India. This allows them to bring their innovative FDA-cleared SaMD screening solution for cancer worldwide.
Combining their liquid biopsy with Guardant Infinity – a data-driven SaMD – can improve accuracy and speed up the cancer screening process. First, the solution considers both genomic and epigenomic profiling for an all-around insight. It then relies on methylation technology to cover more tumor signal.
Bringing both data and Healthtech to life, Guardant Health’s solutions are used by many facilities, from small to big.
Other information:
- Location: Singapore, China, Japan, and India
- Company size: 1000 – 5000 employees
- Security and Compliance Certification: HIPAA, FDA
- Best for: Healthcare organizations of all types, Lab research, Biopharma
- Social proof: over 500,000 tests performed by 12,000 doctors
Harrison
While Guardant focuses on data, Harrison takes a proactive approach in using AI for clinical diagnosis and treatment. Their goal is to help facilities worldwide fight health talent shortage and provide timely care to patients.
The first SaMD solution is Annalise which can support decision-making for chest X-rays and CT scan with up to 124 findings. The second one is Franklin – an AI tool for pathology. Both solutions of this software as a medical device company is not used only in APAC or Asia but also Europe and American.
Other information:
- Location: Australia
- Company size: 50-200 employees
- Best for: Healthcare organizations of all types, Radiologist, Pathologist
- Social proof: Used by 50% of radiologist in Australia
Want to customize a SaMD? Here’s how to find a partner
For a highly-regulated software like SaMD, choosing a partner that can build what you need while navigating quality standard can be a challenge.
These are some criteria to keep in mind.
How they deal with regulatory standard
So far, we’ve learnt that the US and EU impose different standards and risk categories for SaMD solution. But that’s not all. Some international institutions, such as IEC (International Electrotechnical Commission) and IMDRF (International Medical Device Regulators Forum) also have their own evaluation.
Other standards, such as GDPR, HIPAA, or ISO can also be used to qualify the security and data protection of a Software as a Medical device company and solution.
So, a good partner must:
Understand your chosen standard’s requirement and how to acquire them. They can provide the right document and help your submission process faster and easier, as the total process can take from 8 months to years. For example, this is the requirement for a SaMD pre-market submission by the FDA in the US. And this is the requirement by EU MDR.
A plus if your partner can:
- Consult you on how to improve your submission. This would require an in-house legal expert or a custom SaMD company that has worked on many healthcare projects with better hands-on experience.
- Navigate multiple standards at once. As you might want to scale your SaMD solution in the future, having this mindset right from the start will prevent you from multiple iterations and losing money.
Practical tips:
For countries with no clear regulation on the use of SaMD, you can follow the international criterion or use related local regulation in Healthtech, Data in Healthcare, Digital Health, or Telehealth. This is how Synodus does it to ensure quality and security, especially for clients in Asia
Test their ability to deliver
Team quality: Although a big team means enough resources for your project from start to finish, it’s not everything. You should look into the number of experts or developers that can understand the healthcare industry, let alone SaMD.
Practical tips:
Ask how many seniors and mid-level experts your partner can provide. For example, 75% of Synodus developers are mid-level to senior, which is a sign that many clients trust us to handle the complex of SaMD.
Customization ability: As most SaMD rely on complex technologies, a partner who is well-versed in IoT, Data analytics, Cloud computing, or AI is needed. They can then ensure your solution is usable, functionable, and high-performing.
Focus on User experience: Keep in mind that not every doctor and healthcare staff are tech-savvy. Your SaMD needs to be easy to navigate. It should be able to provide as much data in an intuitive way as possible, so doctors can make accurate decisions quicker.
Practical tips:
Find custom software as a medical device company that understands UI/UX. You can look into their relevant portfolio for visual showcase.
Their partnership ethics
Most companies neglect this when searching for a tech partner. Although technical skill is super important, this criterion, on the other hand, decides if your partnership is effective and long enough.
Your partner shouldn’t treat SaMD development as a one-time transaction but a joint effort. Meaning that, they should be able to maintain clear communication and prioritize a win-win approach that values long-term goals.
A plus if they can balance cost and quality. Why? Having a cost-effective mindset will keep your projects on budget and on time. Avoid working with a SaMD company that has foggy pricing structure and unwilling to commit time or quality.
Practical tips:
Many vendors use Agile and Time & Materials pricing model. But this can be quite challenging to accurately estimate price, time, and quality.
Because of this, Synodus employs a different approach. We use a Fixed cost. If we don’t deliver as expected, we take all the risk without you paying for the extra time like T&M.
For quality, we only use Agile in the initial planning phase for better flexibility. Once moving onto the development, we use Waterfall to control deadlines and quality.
The key is to find a way that works best for your current needs. Agile is effective if being used right, and so is Waterfall and Fixed cost.
Wrapping up
We hope that our list of top Software as a Medical device companies across the US, EU, Asia and Australia can help you select the right product and service. Whether you are a Healthtech startup, established healthcare providers, or small size facility, a suitable partner can help you a lot in your venture.
How useful was this post?
Click on a star to rate it!
Average rating / 5. Vote count:
No votes so far! Be the first to rate this post.




